9.6 Extraction of metals
1. Reactivity series and extraction
Less reactive metals can be found in nature as elements, and they can be extracted by displacement reaction using carbon from their ores.
More reactive metals cannot be found in nature as elements. They are ores in nature normally. Pure reactive metal can be extracted by electrolysis.
The diagram below is to show the reactivity series for several metals, and metals which are less reactive than carbon can be extracted by displacement reaction, and reactive can be extracted by electrolysis only.
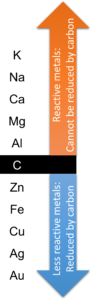
2. Extraction of iron from hematite
Iron can be extracted from hematite in the blast furnace.
(a) the burning of carbon (coke) to provide heat and produce carbon dioxide
C + O2 → CO2
(b) the reduction of carbon dioxide to carbon monoxide
C + CO2 → CO
(c) the reduction of iron(III) oxide by carbon monoxide
Fe2O3 + 3CO → 2Fe + 3CO2
(d) the thermal decomposition of calcium carbonate /limestone to produce calcium oxide
CaCO3 → CaO + CO2
(e) the formation of slag
CaO + SiO2 → CaSiO3
The major impurties in iron ore is sand (SiO2). Step d and e are used to remove sand.

3. Extraction of aluminium from bauxite
The ore of aluminium is bauxite, and aluminium can be extracted by electrolysis. The process involves the following stages:
(a) Tha bauxite is treated with sodium hydroxide in a refinery to obtain pure aluminium oxide (alumina). The alumina produced is shipped to the electrolysis plant.
(b) The purified aluminium oxide is dissolved in molten cryolite (another ore of aluminium). Dissolving the aluminium oxide in cryolite lowers the operating temperature. (The melting point of aluminium oxide is 2030 oC. This is reduce to 900-1000 oC by dissolving the aluminium oxide in cryolite.) The cryolite thus provides a considerable saving in energy costs.
(c) The molten mixture of aluminium oxide and cryolite is electrolyzed in a cell fitted with graphite electrodes (Figure below).
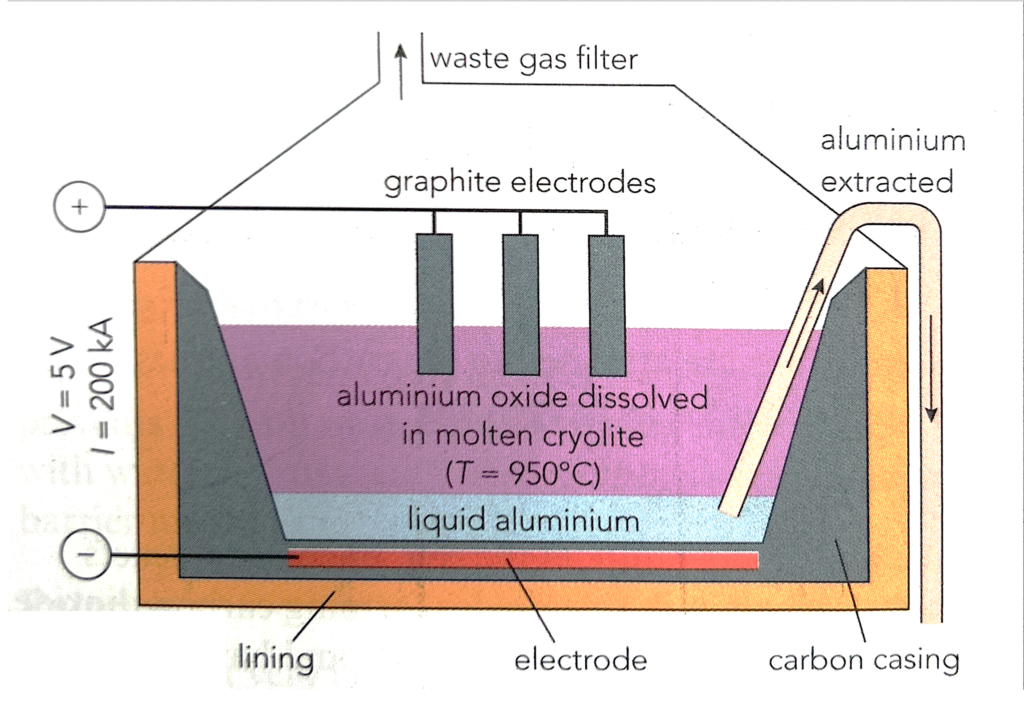
At the high temperature in the cell, the oxygen reacts with the carbon in the electrodes forming carbon dioxide. The carbon anodes slowly burn away and have to be replaced frequently. The cryolite is not used up by the electrolysis and so only alumina needs to be added to keep the process running