5.1 Endothermic and exothermic reactions
1. Basic concepts
System and surroundings: The object of the study is the system, and the rest are surroundings. For example: when we study how the heat change for a neutralization reaction, the reaction is system, and the solution where the reaction took place in is the surroundings.
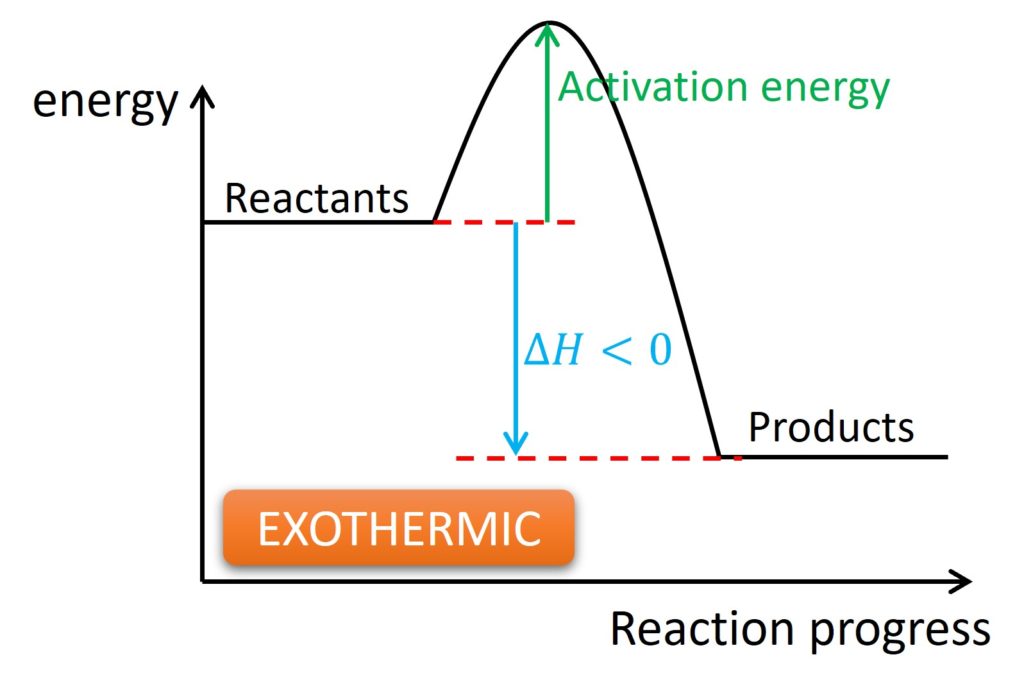
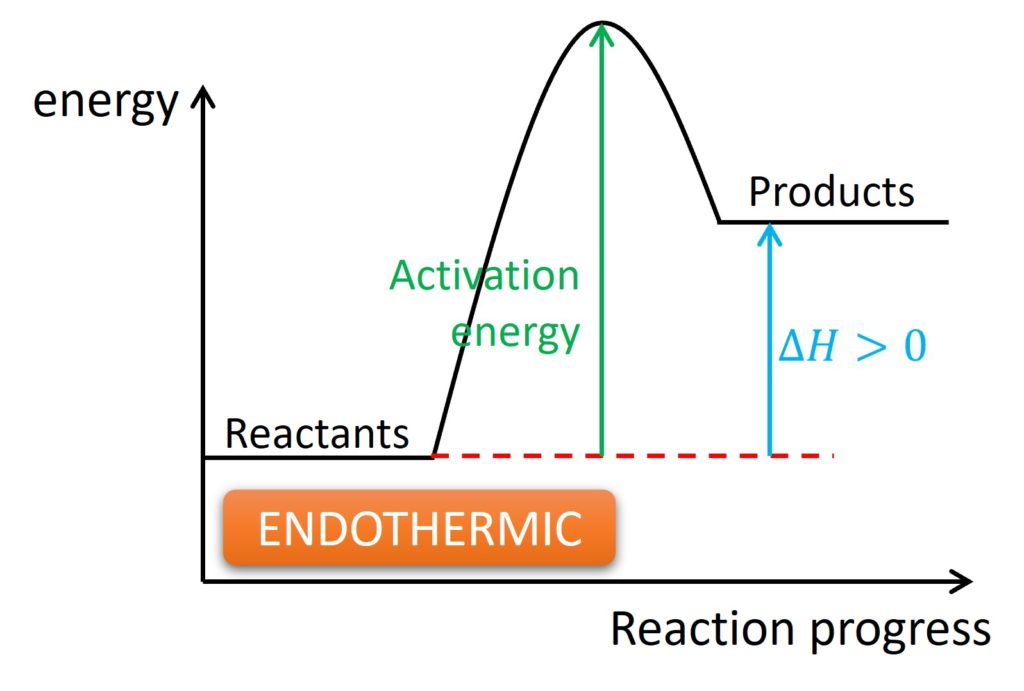
Exothermic and endothermic: They are adjectives and were used to describe the heat change during a reaction. Exothermic represents the heat released by a system, and the temperature of surroundings will be increased. Endothermic represents the heat absorbed by the system, and the temperature of surroundings will be decreased.
One notice is the temperature is always for surroundings. It is impossible for us to measure the temperature of a system.
4. Bond energy
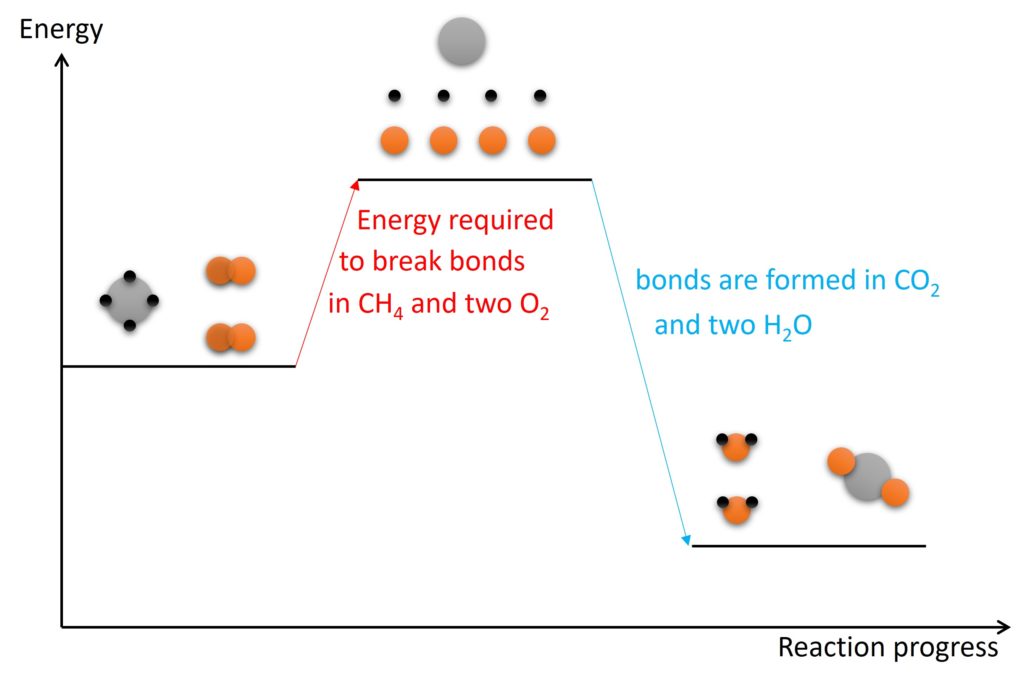
Bond broken is endothermic process and bond forming is exothermic process. The value of energy change, ∆H, should consider both bond broken and bond forming. If energy required for bond broken is greater than energy released when bond formed, the reaction is endothermic. Oppositely, if energy required for bond broken is smaller than energy released when bond formed, the reaction is exothermic.
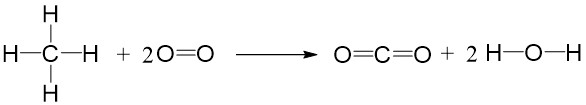
The energy level diagram above shows the process of bonds breaking and forming during the combustion of CH4. The energy required to break bonds in CH4 and O2 is smaller than energy released when bonds formed in products. So the energy change (or enthalpy change) for the combustion of CH4 is exothermic and ∆H is a negative value.
The data of energy for bond breaking and forming are listed in the following table.
| Bond | Bond energy, kJ mol-1 | Bond | Bond energy, kJ mol-1 |
|---|---|---|---|
| C-H | 414 | C=O | 803 |
| O=O | 498 | O-H | 464 |
Calculate the enthalpy change of combustion of methane (CH4).
Bond energy in reactants = 4X(C-H) + 2X(O=O) = 4X414 + 2X498 = 2652 kJ mol-1
Bond energy in products = 2X(C=O) + 4X(O-H) = 2X803 + 4X464 = 3462 kJ mol-1
∆H = 2652 – 3462 = -810 kJ mol-1