1. The problem of corrosion
When a metal is attacked by air, water or other surrounding substances, it is said to corrode. Most metals corrode. They undergo a chemical reaction with oxygen and other gases in the air to form compounds that collect on the surface of the metal. Some very reactive metals such as sodium and potassium need to be stored under oil to keep them away from air and water. Others such as calcium will slowly corrode away to powder over time.
In the case of iron and steel, the corrosion process is also known as rusting. Rusting is a serious economic problem. Large sums of money are spent each year replacing damaged iron and steel structures or protecting structures from such damage. Rust is a red-brown powder consisting mainly of hydrated iron(III) oxide (Fe2O3•xH2O). Water and oxygen are essential for iron to rust. The problem is made worse by the presence of salt; seawater increases the rate of corrosion as can be seen on countless shipwrecks around the world. Acid rain also increases the rate at which iron objects rust.

Experiment to identify the conditions for rusting:
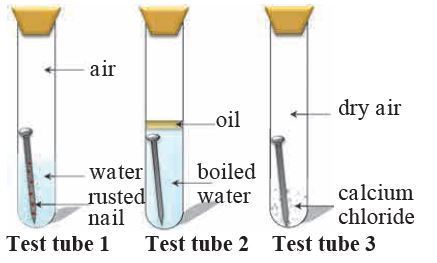
The diagram above shows a series of experiments to determine the conditions for rusting. In the first test-tube, both air(oxygen) and water are present in it, and the nail was rusted finally. In the second test-tube, only water was contacted with iron nail. The oil layer can prevent the air to dissolve in water. In the third test-tube, only dry air was presence in it. The purpose of calcium chloride is to absorb water in air. The iron nails in the second and third test-tubes cannot be rusted. It is concluded that the conditions for rusting is air and water (wet air).
Aluminium is more reactive than iron, but it does not corrode in the damaging way that iron does. Both metals react with air. In the case of aluminium ,a very thin single layer of aluminum oxide forms, which sticks strongly to the surface of the metal. This micro-layer seals the metal surface and protects it from further attack. Aluminium is a useful construction material because it is protected by this layer. The protective layer of aluminium oxide can be made thicker by electrolysis (anodised aluminium).
2. Rust prevention
1) Barrier methods
Rust can be prevented by a coating of material that prevents the iron or steel from coming into contact with water and oxygen. These methods are known as barrier methods.
Painting

Painting is a very common method of protection, and is used for objects ranging in size from ships and bridges to garden gates. Some paints react with the metal to form a stronger protective layer. However, generally, painting only protects the metal for as long as the paint layer is unscratched. Regular repainting is often necessary to keep this protection intact.
Oiling and greasing

The oiling and/or greasing of the moving parts of machinery forms a protective film, preventing rusting. Again, the treatment must be repeated to continue the protection.
Plastic coatings

Plastic coatings are used to form a protective layer on items such as refrigerators and garden chairs. The plastic poly(vinyl chloride), PVC, is often used for this purpose.
2) Galvanising
An object may be coated with a layer of the more reactive metal, zinc. This is called galvanising. It has the advantage over other barrier methods in that the protection still works even if the zinc layer is badly scratched. Galvanising is in fact both a barrier method of protection, while the zinc layer in unbroken, and a form of sacrificial protection, if the zinc layer is scratched or broken. If the zinc layer is broken it is still corroded away in preference to the iron as zinc is a more reactive metal than iron.
The zinc layer can be applied by several different methods. These include electroplating or dipping the object into molten zinc. The bodies of cars are dipped into a bath of molten zinc to form a protective layer.

3) Sacrificial protection
Sacrificial protection is a method of rust prevention in which blocks of a reactive metal are attached to the iron surface. Zinc or magnesium blocks are attached to oil rigs and to the hulls of ships. These metals are more reactive than iron and will be corroded in preference to it: they ‘sacrifice’ themselves so the iron does not rust.

Underground gas and water pipes are connected by wire to blocks of magnesium to obtain the same protection. In all cases, an electrochemical cell is set up. The metal blocks lose electrons in preference to the iron and so prevent the iron forming iron(III) oxide. The more reactive metal oxidises more readily than iron, so it ‘sacrifices’ itself while the iron does not rust.
发表回复