1. Reactivity series
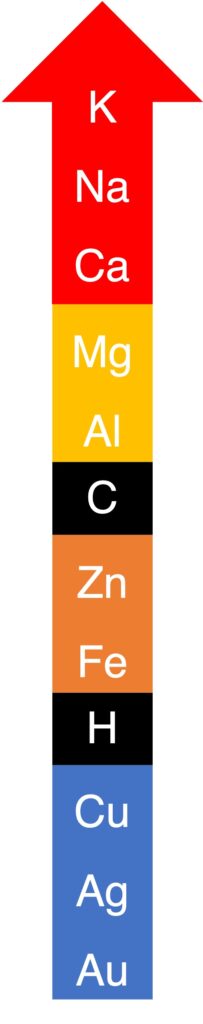
The reactivity of metals are shown above.
Based on this reactivity series, several results can be deduce.
1. Extraction of metal
Metals below H can be extracted by hydrogen; metals between C and H can be extracted by carbon; metals above C can be extracted by electrolysis due to extremely reactivity.
2. displacement reaction
More reactive metal can displace less reactive metal from its compound. For example,
Zn + CuSO4 → ZnSO4 + Cu
or ionic equation: Zn + Cu2+ → Zn2+ + Cu
because Zn is more reactive than Cu
You are required to deduce the reactivity series of metals based on the results of experiment. For instance,
X, Y and Z are metals, and all of them can form ions with charge of 2+. Based on the following reactions, arrange by increasing reactivity.
reaction 1: X + YSO4 → XSO4 + Y
reaction 2: X + ZSO4 → XSO4 + Z
reaction 3: Y + ZSO4 → no reaction
X is more reactive than Y from reaction 1.
X is more reactive than Z from reaction 2.
Y is less reactive than Z from reaction 3.
Result: Y < Z < X
3. reactions with acid and water
K, Na, Ca and Mg are too reactive, and they can react with water. The first three metals are extremely reactive and can react with cold water, and Mg can react with hot water (steam).
2K(s) + 2H2O(l) → 2KOH(aq) + H2(g)
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
Ca(s) + 2H2O(l) → Ca(OH)2(aq) + H2(g)
Mg(s) + H2O(g) → MgO(s) + H2(g)
Metals above H can react with dilute acids and metals below H cannot react with dilute acids.
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
2Al(s) + 6HCl(aq) → 2AlCl3(aq) + 4H2(g)
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
Fe(s) + 2HCl(aq) → FeCl2(aq) + H2(g)
Cu(s) + 2HCl(aq) → no reaction
Ag(s) + 2HCl(aq) → no reaction
Au(s) + 2HCl(aq) → no reaction
2. Reactivity of Aluminium
Aluminium is reactive in the reactivity series, but you will see that aluminium reacts less strongly than would be expected. This occurs because aluminium reacts easily with oxygen in the air to form a thin layer of aluminium oxide (Al2O3) that extends over the whole metal surface.
4Al(s) + 3O2(g) → 2Al2O3(s)
This unreactive oxide layer is very firmly attached to the metal and does not flake off. The layer acts as a protective coating, slowing the reaction. Only when the oxide layer is removed is the true reactivity of aluminium observed. It is possible to remove the protective oxide layer and aluminium then shows a reactivity with acids just below that of magnesium. The reactivity of aluminium (when not covered in a layer of oxide) puts it in a position within the reactivity series just below magnesium, but above zinc.
发表回复