2. Enhance the properties of alloy
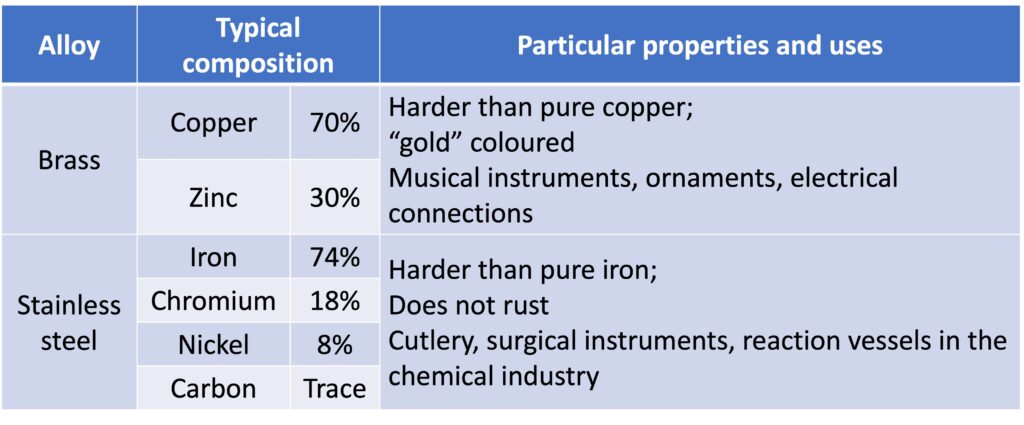
Alloys are harder and stronger than the pure metals and are more useful. For example, stainless steel in cutlery because of its hardness and resistance to rusting.
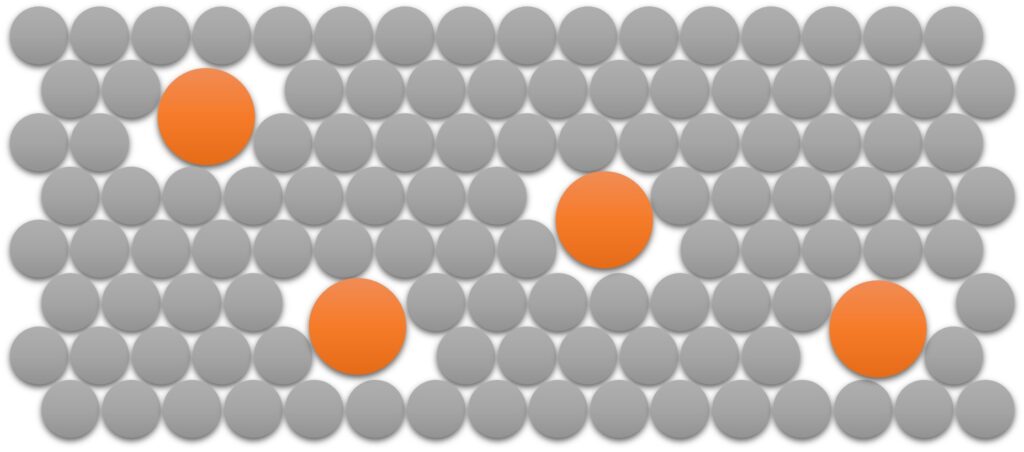
When a metal is alloyed with a second metal, the different sized atoms make the lattice structure less regular. The crystal lattice of the main metal present is disrupted. Figure below shows how the presence of the ‘impurity’ atoms (the larger atoms in this diagram) makes it more difficult for the meta atoms to slide over each other. This makes the alley stronger but more brittle Thant he metals it is made from. The alloy tends to be more brittle than the pure metal as the regularity of the structure has been broken.
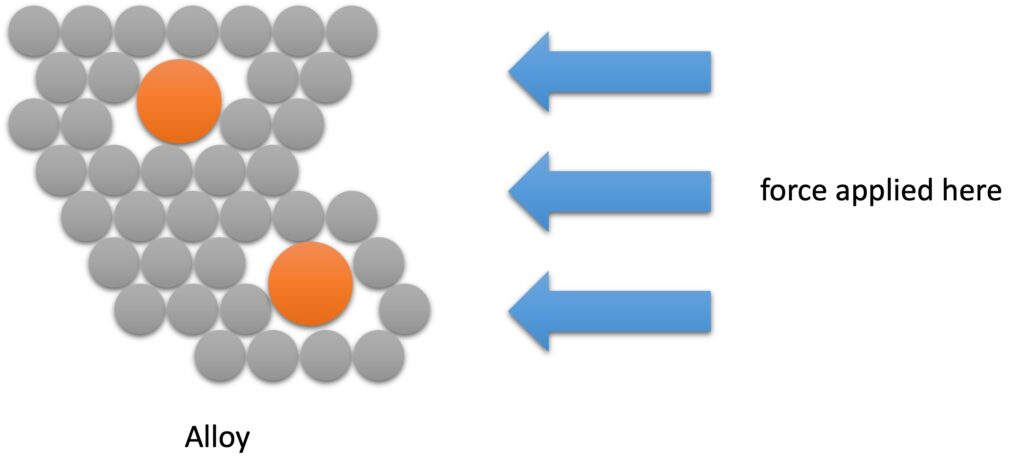
The alley lattice is still held together by metallic bonding with delocalised electrons free to move between the layers of the structure. Alloys, therefore, share the same characteristic physical properties – malleability, ductility, electrical and thermal conductivity – as the metallic elements.
发表回复