All of the noble gases are present in the Earth’s atmosphere. Together they make up abut 1% of the total, though argon is the most common. These gases are particularly unreactive. They were sometimes referred to as the inert gases, meaning they did not react at all. However, since the 1960s, some compounds of xenon and krypton have been made and their name was changed to the noble gases.
The uses of the noble gases depend on their unreactivity. Helium is used in airships and balloons because it is both light and unreactive. Argon is used to fill incandescent light bulbs because it will not react with the filament even at high temperatures. The best-known use of the noble gases is, perhaps, its use in “neon” lights. The brightly colored advertising lights work the an electric discharge takes place in a tube containing small amount of a noble gas. Different gases give different colors.

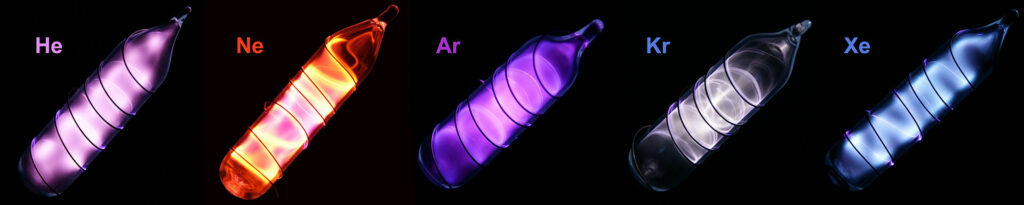
The atoms of the noble gases do not combine with each other to form molecules or any other form of structure. Their melting points and boiling points are extremely low. Helium has the lowest melting point of any element and cannot be solidified by cooling alone (pressure is also needed).
All these properties point to the atoms of the noble gases being particularly stable:
- the electron electronic configurations of the atoms of the noble gases are energetically very stable
- this means that they do not react readily with other atoms
- in many situations where atoms of other elements bond or react chemically, they are trying to achieve the energetically stable arrangement of electrons found in the noble gases.
The elements of Group VIII lie between the two most reactive groups of elements (Groups I and VII) in the Periodic Table. Indeed, it is their closeness to this group with stable electron arrangements that makes the alkali metals and the halogens so reactive. The can fairly easily achieve a noble-gas electron structure. The Group VII elements gain or share electrons and the Group I elements lose electrons to reach a noble gas electronic configuration.
发表回复