The elements between Group II and Group III are called transition elements. Their properties make them among the most useful metallic elements available to use. They are much less reactive than the metals in Groups I and II. Many have excellent corrosion resistance, for example chromium. The very high melting point of tungsten (3410) led to its use in the filaments of incandescent light bulbs.
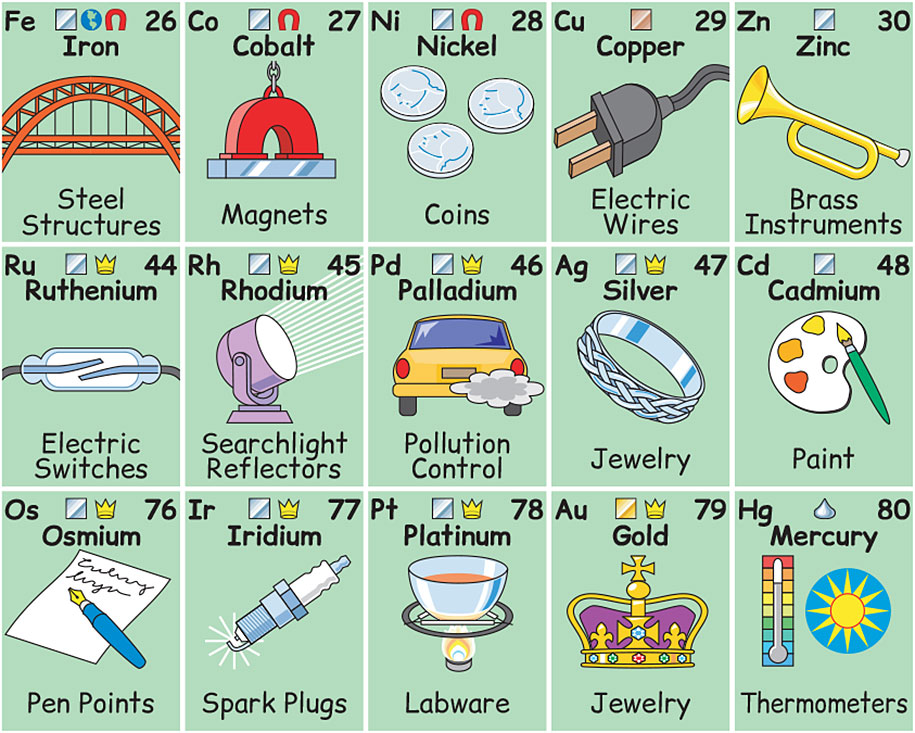
Many familiar objects are made from transition metals. The figure above shows some application of transition metals in our daily life.
These general properties mean that the transition metals are useful in a number of different ways. In addition, there are particular properties that make these metals distinctive and useful for more specific purposes. One important feature of transition metals is that their compounds are often colored.

General features of transition metals:
- they are hard and strong
- they have high density
- they have high melting and boiling points.
Distinctive properties of transition metals:
- many of their compounds are coloured
- the transition metals, or their compounds, often act as catalysts. Iron, for example, is used as a catalyst in the Haber process for making ammonia.
- they often show more than one valency (variable oxidation number) – they form more than one type of iron. For example, iron can form compounds containing iron(II) ions (Fe2+) or iron(III) ions (Fe3+).
- These metals can often form more than one type of oxide because of the different oxidation number they can show. The oxides of the lower oxidation number are basic ionic oxides, eg copper(II) oxide and chromium(II) oxide. However, the oxides of the highest oxidation number, eg chromium(VI) oxide, tend to be covalent and produce acidic solutions in water. Chromium(III) oxide (Cr2O3) is similar to aluminum oxide (Al2O3) in being an amphoteric oxide.
发表回复