1. The structure of periodic table
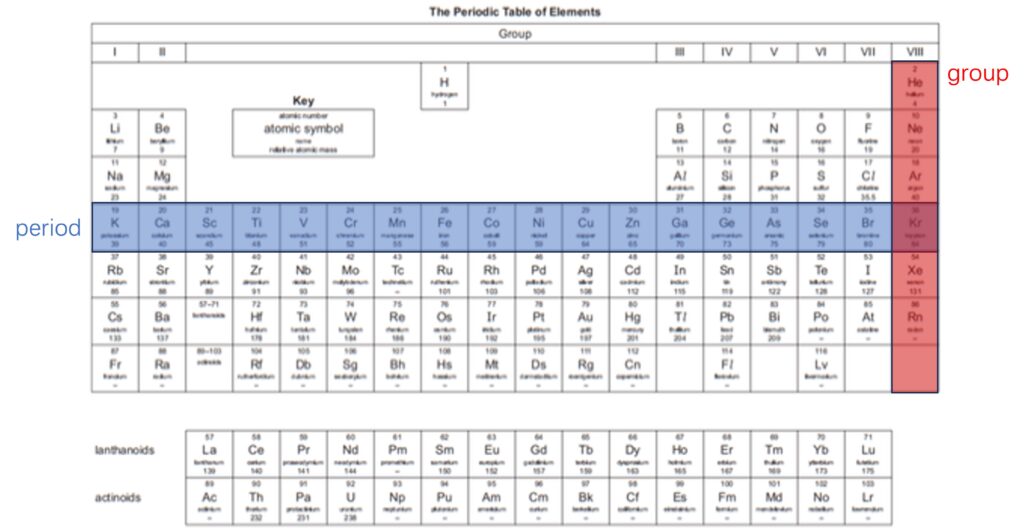
All modern versions of the Periodic Table are based on the table proposed by Mendeleev in 1869, and one typical one is shown above.
In the Periodic Table:
– elements are arranged in order of increasing proton (atomic) number;
– vertical columns of elements with similar chemical properties are called groups;
– horizontal rows are called periods.
The position of one element can predict the electron arrangement of it. The group number indicates the number of electrons at outer shell, which decides chemical properties of this element. The period number shows how many shells of electrons the atom has. For instance, sodium (Na) is located at Group I and Period 3. This information tells us that sodium has one electron at its outermost shell, and three electronic shells can be found around its nucleus.
2. Metals and non-metals
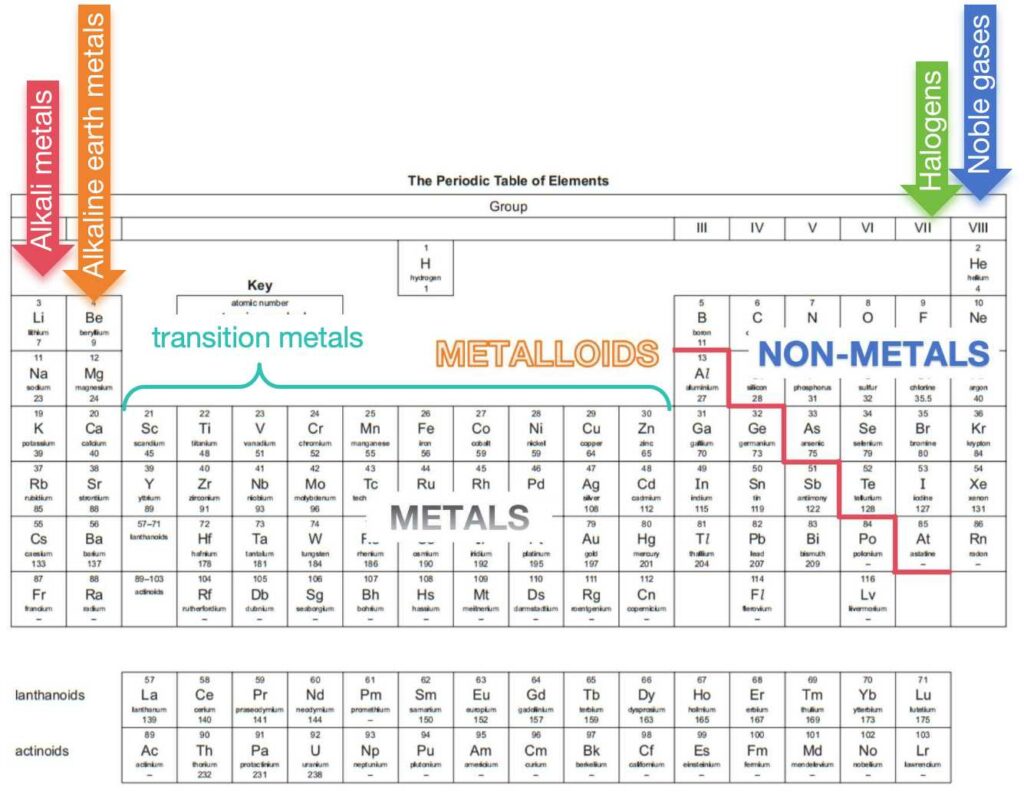
The zig-zag line (red line in the Periodic Table above) can separate two regions. All metals are at left side and all non-metals are at right side. Some elements around zig-zag line are metalloids which means they have same of the properties of metals and others that are more characteristic of non-metals. One notice is that all metalloids are around this zig-zag line, but it does not mean all elements around this line are metalloids. For example, Aluminium is around this line, but it is a metal.
Across the period, it shows an increasing non-metallic characteristics and a decreasing metallic characteristics. Down the group, it shows a decreasing non-metallic characteristics and an increasing metallic characteristics.
The elements present in Group I to VIII of the Periodic Table are sometimes known as the main-group elements. Between Groups II and III of these main groups of elements is a block of metals known as the transition elements (or transition metals).
| Group | Common name |
| I | Alkali metals |
| II | Alkaline earth metals |
| between II and III | Transition metals |
| VII | Halogens |
| VIII | Nobel gases |
3. Predict ions from the Periodic Table
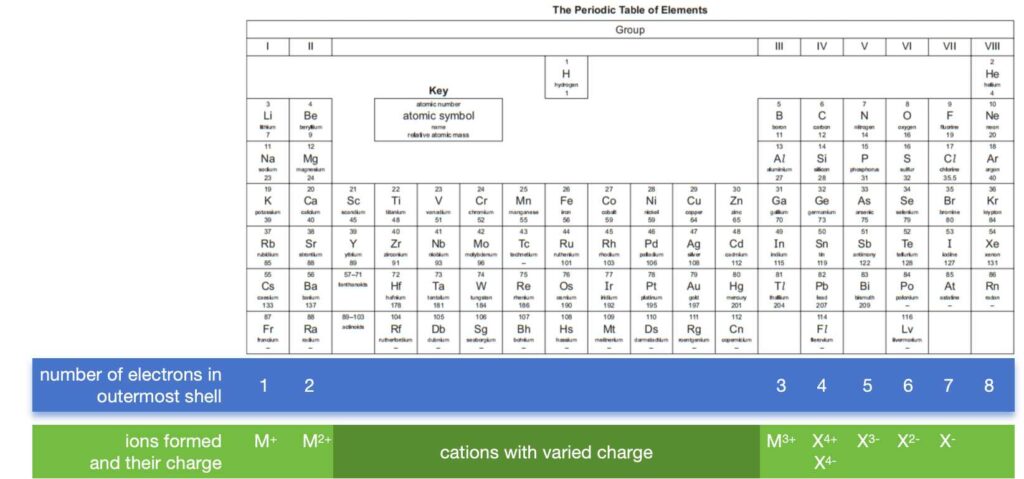
Ions and their charged can be formed can be deduced from the position on the Periodic table. Actually, it depends on the number of electrons at outermost shell.
Elements in the same group have same number of electrons at outermost shell, so that they can form same charged ions. For examples, Li, Na and K are group I elements and have one electron at outermost shell only. All of them will form cations, which are positively charged ions, and with a charge of 1+.
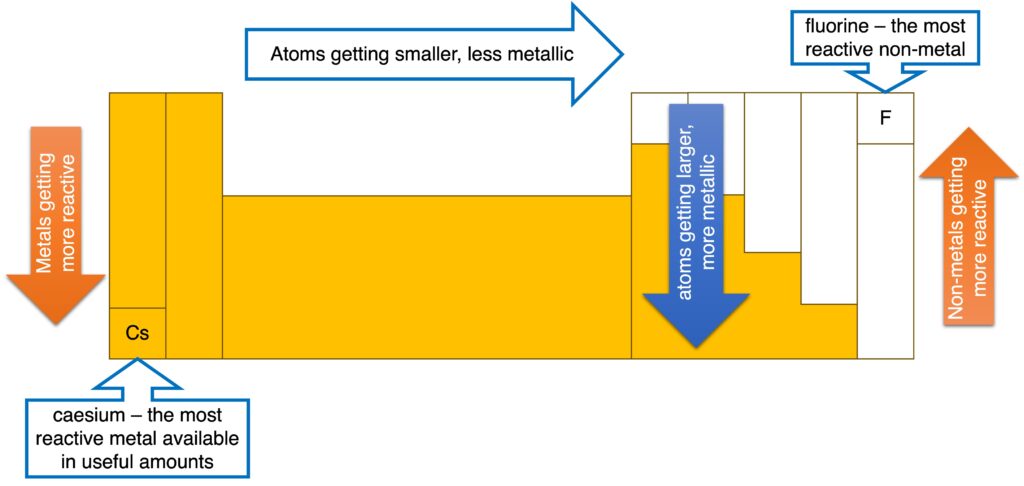
4. Periodicity
The Periodic Table shows periodicity which means some repeating patterns of physical and chemical properties can be shown across the periods and down the groups.
发表回复