1. Tests for the presence of water
The presence of water can be tested by the color change of some anhydrous salt, such as cobalt (II) chloride and copper(II) sulfate.
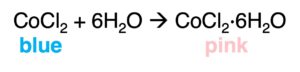
or
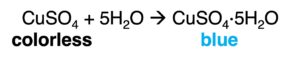
2. Test for the purity of water
All pure substances have a fixed melting points and boiling points. The melting point of pure water is 0 ℃ and boiling point is 100 ℃ under one atmospheric pressure. The impurity in water will lower the melting point and raise the boiling point.
3. Distilled water
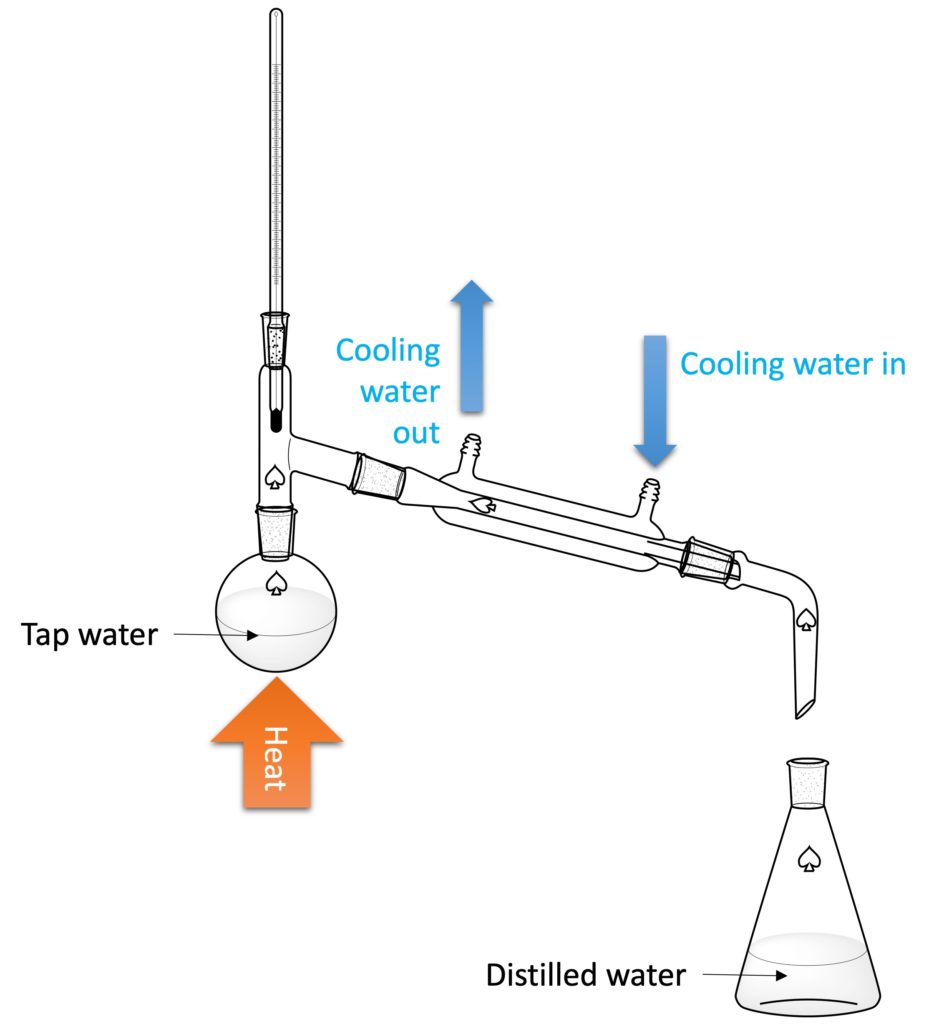
The distilled water can be collected by distillation. The tap water can be place in the round-bottom flask. With the increasing temperature, the water vapor will pass through condenser and condensed to water (liquid) again. Finally, distilled water can be collected in the conical flask. The impurities (such as metal ions) will be left in the round-bottom flask.
Water is one of the commonest chemicals in the experiment. It can be used to wash the apparatus, as the solvent for some chemical reactions, and so on. Experiments required distilled water rather than tap water because some substances dissolved in the tap water may interfere with experiments giving unwanted side reactions.
4. Substances in water are beneficial
(1) Dissolved oxygen
All livings are required oxygen gas. The dissolved oxygen in water can support aquatic life.
Oxygen enters the water either as a result of photosynthesis by aquatic plants or through diffusion of oxygen from the air.
Oxygen is removed from the water by respiration plants and animals.
The amount of oxygen dissolved in water depends on temperature and whether it is saltwater or freshwater.
(2) Metallic compounds
Some metal compounds precede essential minerals for life. There are a large range of metals that are needed in trace amounts to support good health. Such as Group I metals, Group II metals and a range of transition metals ions (iron, cobalt, nickel, copper, zinc and chromium).
Calcium, for example, supports the health of teeth and bones while iron is needed in the production of hemoglobin (for use in red blood cells).
5. Substances in water are harmful
(1) Metallic compounds
Besides the metallic ions above, some metallic compounds are toxic. Heavy metals such as lead and mercury can enter water systems from a variety of sources including mining, metal smelting, waste disposal, corrosion and metal processing plants. Lead can cause liver and kidney damage, and mercury has been linked to damage of the nervous system.
(2) Plastics
Plastics are polymers and they are used throughout our daily life. Plastics are insoluble in water and so can be easily removed. The issues being caused by plastics are due to the volume of waste being released.
Poor disposal of these materials combined with a lack of biodegradability has resulted in polymers being released into waterways. As they do not breakdown, they can rapidly accumulate. The impacts of accumulated plastics in our oceans and waterways on wildlife can be catastrophic.
There are several aspects to the problem of plastic pollution of the oceans and waterways:
– Large sea creatures ad sea birds can be trapped by discarded fishing nets.
– Large scale debris such as intact plastic bags can be infused as prey such as jelly fish and so consumed by whales, turtles and large fish. Once consumed these plastic items can block the digestive system and ultimately lead to death.
(3) Sewage
Sewage contains harmful microbes which cause disease.
Sewage is usually carried by underground pipes (sewers) and taken to wastewater treatment plants to remove the harmful materials. Any solids can be filtered or digested and the treated liquid (effluent) returned into rivers or the sea. Leaks of sewage into drinking water can happen during natural disasters such as extreme weather events or earthquakes. When this happens, harmful microbes enter the drinking water spreading diseases such as diarrhea, cholera, dysentery, typhoid and polio.
(4) Nitrates and phosphates
Nitrates and phosphates lead to deoxygenation of water and damage to aquatic life.
NPK fertilizers are used to increase crop yield by adding three essential plant nutrients nitrogen (N), phosphorus (P) and potassium (K) to the soil. They are made from water-soluble compounds (salts) that are easily absorbed through the roots of plants. These water-soluble compounds create problems if there is heavy rain after the fertilizer has been spread onto the crops. Under these conditions, instead of being taken in by the plants the fertilizer will be washed over the surface of the soil and into waterways. This process is called run-off.
6. Treatment of the domestic water
Tap water, the domestics water supplied to our house, undergoes several purification steps from the point it is collected to the point it is delivered. In many countries, domestic water is taken from lakes and reservoirs. The first step in purification is to remove large insoluble objects such as rocks, plastic bags and branches, in a process known as screening. The water is then taken to a sedimentation tank. In the sedimentation tank the soil and sand will drop to the bottom of the tank (as sediment).
The next step in the process is to filter the water to remove smaller insoluble particles. The water is often passed through a very find sand to filter out these particles.
Water may contain dissolved organic compounds that can cause the water to have an unwanted odor or taste. The organic compounds, often present in exceptionally low amounts, can be removed by using an activated carbon filter.
Before water is distributed to homes, the final step is disinfection. Disinfection is needed to kill harmful waterborne microbes such as bacteria that can cause disease. Different countries use different methods to disinfect water but one of the most common and effective methods is to add small amounts of chlorine to the water. Typically, chorine is added at a concentration of 2-3 mg/dm3.
发表回复