1. Methods to prepare salts
The salt in chemistry always consisted by cation and anion, and should be an ionic compound. But it does not mean all ionic compounds are salts. Sodium hydroxide (NaOH) is a base.
Reactions can be used to prepare salts including: neutralisation, single replacement reaction and precipitation, etc.
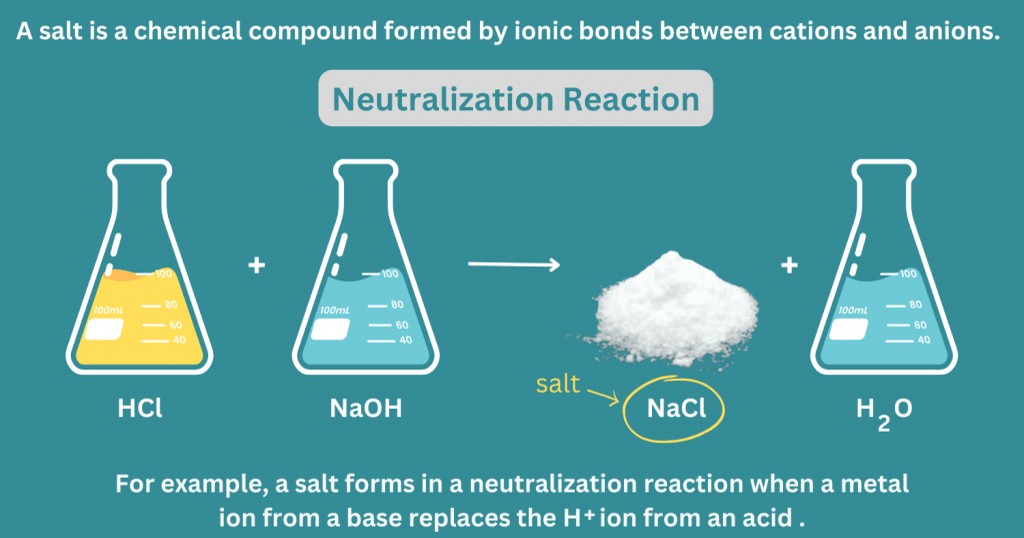
- An acid and a base react via a neutralization reaction.
Example: HCl + NaOH → NaOH + H2O - An acid reacts with a basic oxide (metallic oxide).
Example: 2HNO3 + CuO → Cu(NO3)2 + H2O - A base reacts with an acidic oxide (nonmetallic oxide).
Example: 2NaOH + CO2 → Na2CO3 + H2O - A salt which is insoluble in water by double replacement reaction (precipitation).
Example: Pb(NO3)2(aq) + Na2SO4(aq) → PbSO4(s) + 2NaNO3(aq) - A reactive metal and acid react.
Example: Mg + H2SO4 → MgSO4 + H2 - A metal reacts with a nonmetal.
Example: 2Na + Cl2 → 2NaCl
The problem in preparation of salts is which reactant should be in limited, and which one should be in excess. The strategy is easy to separate target product from reaction mixture.
2. Names of salts
The name of a salt is consisted by name of cation with name of anion.
For example, NaCl is composed by Na+, which is called sodium ion and Cl–, which is called chloride. So the name of NaCl is called sodium chloride.
(a) names of cations
(i) Monatomic cation (ion produced by one atom)
- For group 1 (Na, K, etc.), 2 (Mg, Ca, etc.) and 3 (Al) metals, they will form the cation which has unique charge.
Na: sodium; Na+: sodium ion
Mg: magnesium; Mg2+: magnesium ion
Al: aluminium; Al3+: aluminium ion - For transition metals, most of them have ions with varied charge. So the Roman numerals should be used to indicate the charge on ion.
Cu+: copper(I) ion; Cu2+: copper(II) ion
Fe2+: iron(II) ion; Fe3+: iron(III) ion - Few transition metals have unique charge as well. The name for these metals obey the first rule.
Zn2+: zinc ion
Ag+: silver ion
(ii) Polyatomic cation (ion produced by more than one atoms)
- Only two polyatomic cations should be known.
NH4+: ammonium
H3O+: hydronium
(b) names of anions
(i) Monatomic anion
- Stem with suffix of –ide
Cl: chlorine; Cl- chloride
发表回复