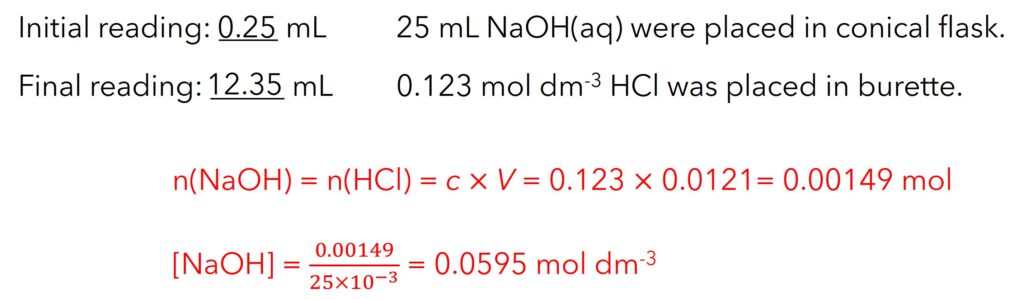IGCSE and A Level Chemistry Learning Website
7.1 The characteristic properties of acids and bases
1. Reactions of acids and bases
a) Acids react with reactive metals
Acid + reactive metal → salt + hydrogen gas
eg:
2HCl(aq) + Zn(s) → ZnCl2(aq) + H2(g)
H2SO4(aq) + Fe(s) → FeSO4
Reactive metals represent metals are above Hydrogen in activity series

b) Acids react with bases
eg:
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
H2SO4(aq) + 2KOH(aq) → K2SO4(aq) + 2H2O(l)
c) Acids react with carbonates
Acid + carbonate → salt + carbon dioxide + water
eg:
CaCO3 + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
Na2CO3(aq) + H2SO4(aq) → Na2SO4(aq) + H2O(l) + CO2(g)
Utilization: Do you still remember how to test the presence specified gases? (H2, CO2, etc?)
a) Bases react with acids
same to reactions involving acids
b) Bases react with ammonium salts
Base + ammonium salt → salt + ammonia gas + water
eg:
NaOH(aq) + NH4Cl(aq) → NaCl(aq) + NH3(g) + H2O(l)
Ba(OH)2(s) + 2NH4Cl(s) → BaCl2(aq) + 2NH3(g) + 2H2O(l)
Acids: proton (H+) donors.
Bases: proton (H+) acceptors.
A strong acid: is an acid that is completely dissociated in aqueous solution.
HCl(aq) → H+(aq) + Cl–(aq)
A weak acid: is an acid that is partially dissociated in aqueous solution.
Notice: dissociations of weak acids or bases should use equilibrium arrow “⇌“.
CH3COOH(aq) ⇌ H+(aq) + CH3COO–(aq)
Some common organic acids and mineral acids are listed in the following table.
| Name | Formula | Strong or weak? | Where found or used |
|---|---|---|---|
| ethanoic acid | CH3COOH | weak | in vinegar |
| methanoic acid | HCOOH | weak | in ant and nettle stings; used in kettle descaler |
| lactic acid | CH3CH(OH)CO2H | weak | in sour milk |
| citric acid | C6H8O7 | weak | in lemons, oranges and other citrus fruits |
| carbonic acid | H2CO3 | weak | in fizzy soft drinks |
| hydrochloric acid | HCl | strong | used in cleaning metal surfaces; found as the dilute acid in the stomach |
| nitric acid | HNO3 | strong | used in making fertilisers and explosives |
| sulfuric acid | H2SO4 | strong | in car batteries; used in making fertilisers, paints and detergents |
| phosphoric acid | H3PO4 | intermediate to strong | in anti-rust paint; used in making fertilisers |
Example: Use HCl to titrate NaOH
Step 1: 25 mL NaOH(aq) were placed in conical flask by pipette;
Step 2: Litmus was dropped into solution. (colour of solution: blue);
Step 3: 0.123 mol dm-3 HCl was placed in burette. Record the initial reading;
Step 4: Identify end-point (or called equivalence point) by colour change (turns purple)
Step 5: Record the final reading.
Step 6: Calculate concentration of NaOH.
IGCSE and A Level Chemistry Learning Website 自豪地采用 WordPress 构建