6.4 Redox
1. Definition
Oxidation: loss of electron
Reduction: gain of electron
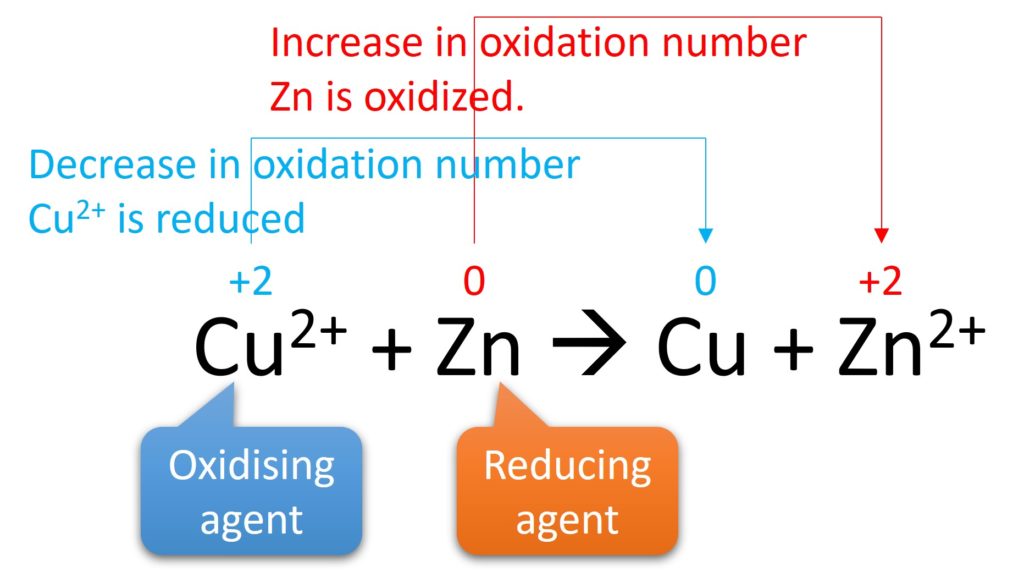
Based on this definition, oxidation and reduction is no longer an individual reaction, they are half-equations. Oxidation and reduction is an overall reaction, which is called REDOX.
Oxidation: Mg ⟶ Mg2+ + 2e–
Reduction: O2 + 4e– ⟶ 2O2-
Overall: 2Mg + O2 ⟶ 2Mg2+ + 2O2-
When oxidation pluses with reduction, the number of electron loss should be equal to the number of electron gained in another half-equation.
Notice:
a) difference among charge, oxidation number and oxidation state
Charge is a real number which is from loss or gain of electron to form a positively charged or negatively charged ion.
Oxidation number (state) is an artificial number. For example, nonmetals share electrons in covalent bond. We assume that the atom which has greater attraction for bonding pair electrons gains these electrons and the atom which has weaker attrition for bonding pair electrons loses these electrons to form charges. But this charges are not real, and we call them oxidation number (state).
Oxidation number is Roman numeral (I, II and III) and Oxidation state is +/- with Arabic numeral (+1, +2, -1, and -2).
b) Rules for oxidation number
For element: Oxidation number = 0;
For ion: oxidation number = charge on ion;
For polyatomic ion, total oxidation number = charge on ions;
For compound, total oxidation number = 0;
In compound:
i) H is +1 with nonmetal and -1 with metal.
ii) O is usually -2; in H2O2 (hydrogen peroxide), O has oxidation number with -1. When O combined with F, O is positive oxidation number.
iii) Group 1 metals always form an oxidation number with +1.
iv) Group 2 metals always form an oxidation number with +2.
v) Other oxidation number can be deduce from rules above.